The rising thirst for electrification or electric mobility is swallowing up rare materials such as lithium, nickel, and cobalt in the production of all those batteries. Electric cars are supposed to help the planet, but could their batteries create the next trash problem? The future seems to be green and electric, but at what cost? Let’s review this article to understand the need for battery recycling for this decade.

What is a Battery?
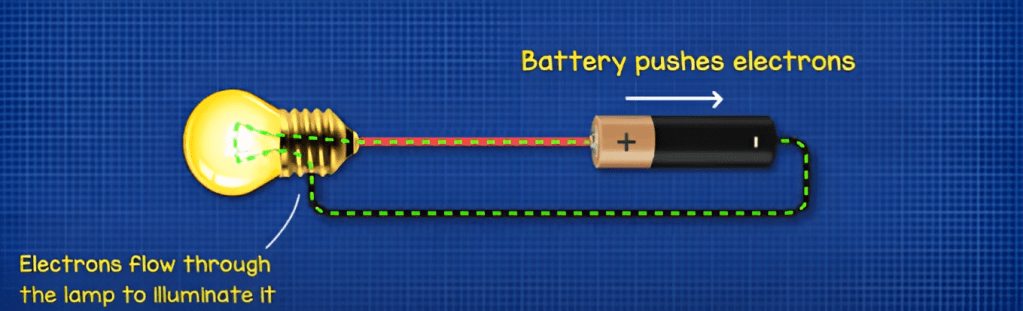
A Battery is an electrochemical device that stores chemical energy and converts it to electrical energy. It consists of one or more electrochemical cells connected together. Each cell contains two electrodes, a positive electrode (cathode) and a negative electrode (anode), which are typically made of different materials.
Batteries are broadly classified into two categories: primary batteries and secondary batteries. Primary batteries also known as disposable batteries, are designed for single-use only, meaning they can be charged and used once. Secondary batteries, also known as Rechargeable batteries, are designed to be charged and discharged repeatedly over multiple cycles. As a result, secondary batteries can be recharged, making them a convenient and sustainable power source for various devices.
Lithium-ion batteries, have become incredibly popular and are widely used in everyday gadgets like smartphones, laptops, tablets, and wearable devices. They are also extensively employed in electric cars and energy storage systems because of their ability to store a lot of energy and last for a long time. From our handheld devices to sustainable transportation, lithium-ion batteries have become the go-to power source, revolutionizing the way we use technology.
What is a Lithium-ion Battery?
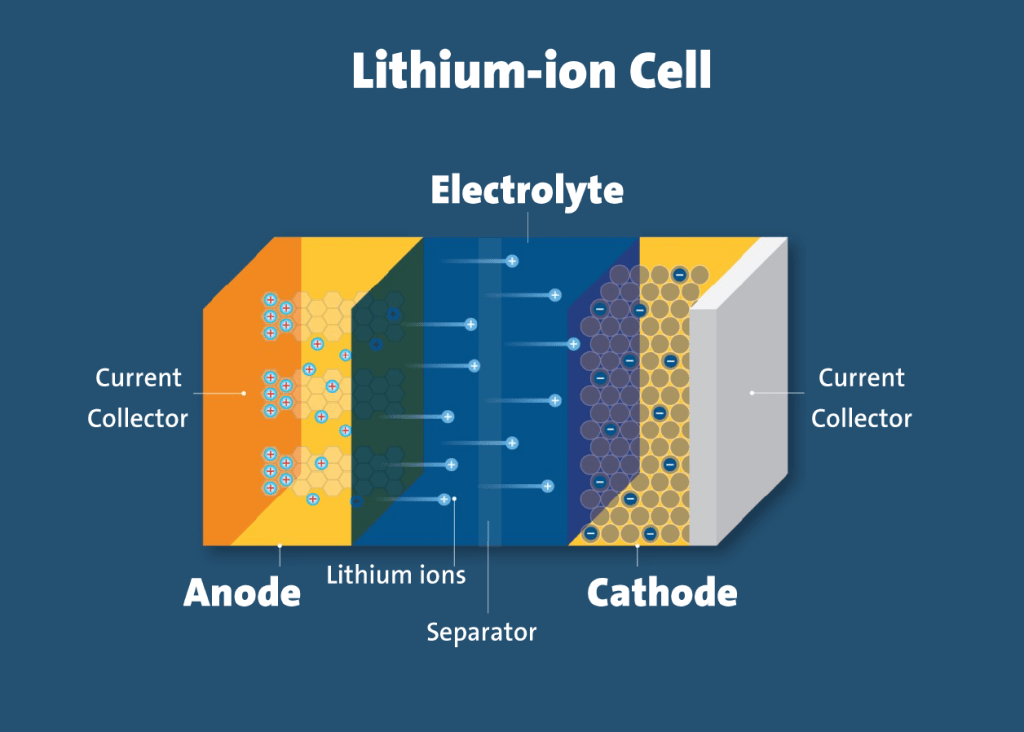
A lithium-ion battery is a type of rechargeable battery that is charged and discharged by lithium ions moving between the negative (anode) and positive (cathode) electrodes. The key elements inside lithium-ion electric car batteries are the anode, cathode, electrolyte and separator. The battery cells in EVs contain roughly 17 pounds of lithium carbonate, 77 pounds of nickel, 44 pounds of manganese, and 30 pounds of cobalt.
The reason why Lithium-ion batteries are preferred over others is because:
1. High energy density: Stores more energy in a compact and lightweight package.
2. Temperature resilience: Performs well in both high and low temperatures.
3. Low self-discharge rate: Maintains energy even when not in use for extended periods.
4. Long cycle life: Endures multiple charge cycles with minimal capacity loss.
Cobalt: The Blood Diamond of Batteries
Cobalt (chemical symbol Co) is a magnetic and lustrous steel grey metal possessing similar properties to iron and nickel in terms of hardness, tensile strength, machinability, thermodynamic properties, and electrochemistry. Cobalt is one of only three naturally occurring magnetic metals (with iron and nickel).
Cobalt is the most valuable metal key component for manufacturing lithium-ion batteries which are used by electric devices and electric cars. Lithium as a metal on its own is very highly reactive and sensitive to moisture and oxygen. Therefore, the positive end (cathode) is made up of Lithium Cathode Oxide that has the chemical formula LiCoO2 which is a stable way of keeping hold of the lithium. So within a battery, the Lithium Cobalt Oxide is the source of Lithium and the cobalt on the oxygen tries to make that compound as stable as possible.
There is a growing focus on the supply risks associated with critical battery materials, including cobalt, in the context of transitioning to electric mobility. Although battery technology and recycling advancements are recognized as strategies to mitigate these risks, their effectiveness in alleviating the global and regional imbalance between cobalt demand and supply is still not well understood.
Battery Recycling Process
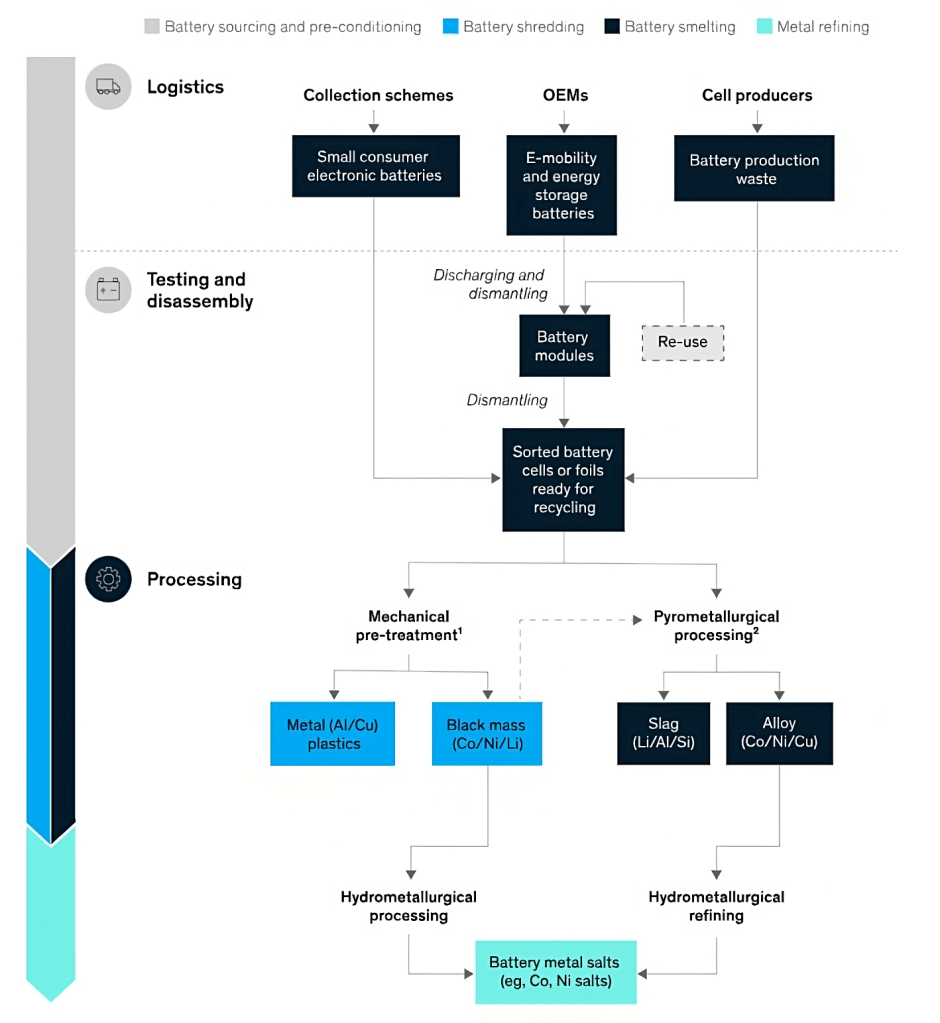
The recycling of lithium-ion batteries typically involves a combination of physical and chemical processes to safely recover valuable materials. The complex assembly and diverse electrode types in lithium-ion batteries pose risks during the recycling process, including explosions, combustion, and the release of toxic gases. To mitigate these dangers, spent lithium-ion batteries are often discharged prior to recycling.
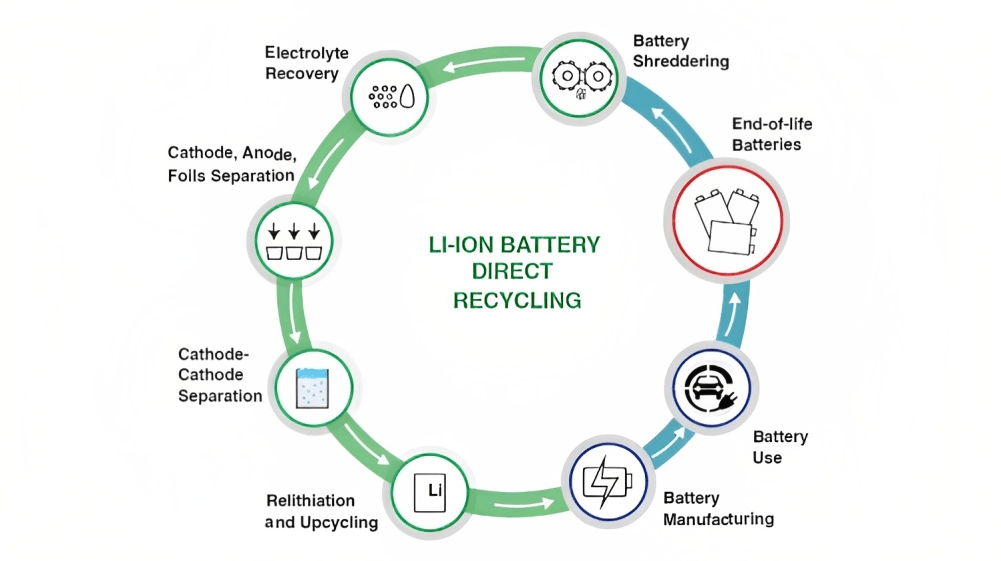
There have been various battery recycling processes been introduced in the market but only a few are preferred and commonly implemented. Let us understand the three basic processes in detail:
1. Physical/Direct Recycling Process
The cycling process for cobalt in electric batteries typically involves several stages. First, the batteries are collected and sorted. Then, they undergo dismantling and mechanical processes to separate the battery components. The cobalt-containing materials, such as cathodes, are extracted and purified. Finally, the extracted cobalt can be reintroduced into the battery manufacturing supply chain. This is the traditional process of recycling the batteries.
2. Hydrometallurgical Processes
Hydrometallurgical processes use aqueous solutions to dissolve and extract metals from Lithium-ion Battery components. The cathode and anode materials are separated and subjected to leaching using appropriate solvents. The dissolved metals can be further processed through precipitation, solvent extraction, and refining to recover valuable materials. Mechanical pretreatment combined with hydrometallurgical processing presents a complex, though viable process, which requires more reagents to achieve high material recovery rates and battery-grade quality products. The hydrometallurgical process involves three steps:
Leaching: The first stage of the process depends on the metal ore being extracted and involves bringing aqueous solutions into contact with the material. Using atmospheric and pressure acid, the most common leaching agent is dilute sulfuric acid.
Solution-based concentration and purification: After leaching, the solvent extraction, precipitation, and simulation processes are used. The leach liquor is concentrated to recover metal ions, while precipitation selectively removes targeted metal compounds or impurities.
Metal recovery: The final stage involves the recovery of salts in metal through cementation and oxidation methods. Metal recovery often yields metals suitable for direct sale as raw materials.
3. Pyrometallurgical Processes
Pyrometallurgical methods involve high-temperature treatments to extract metals from LIBs. The batteries are subjected to thermal processes, such as smelting or roasting, which can help separate the metals from other materials. The recovered metals can then undergo refining and purification. However, only a few raw materials, such as cobalt and nickel, can be recycled. Lithium, aluminum, and manganese end up in the slag and are not recovered, as this is not economically feasible. Pyrometallurgy generally involves three steps:
Roasting: Heating of compounds in air and transforming sulfide ores into oxides, creating gas.
Smelting: Furnaces use it to reduce metals like iron ore, tin, copper, and lead, forming carbon dioxide.
Refining: sorts metals based on their chemical and metallurgical properties. High-temperature smelting in furnaces and electrolytic processes are used to separate metals.
MARKET FOR BATTERY RECYCLING
The global demand for lithium-ion batteries is projected to experience a significant surge over the next ten years. The required capacity is anticipated to increase from approximately 700 GWh in 2022 to around 4.7 TWh by 2030. In 2030, the majority of this demand, around 4,300 GWh, will be driven by batteries used in mobility applications such as electric vehicles (EVs). This trend is not surprising considering the rapid growth of the mobility sector.
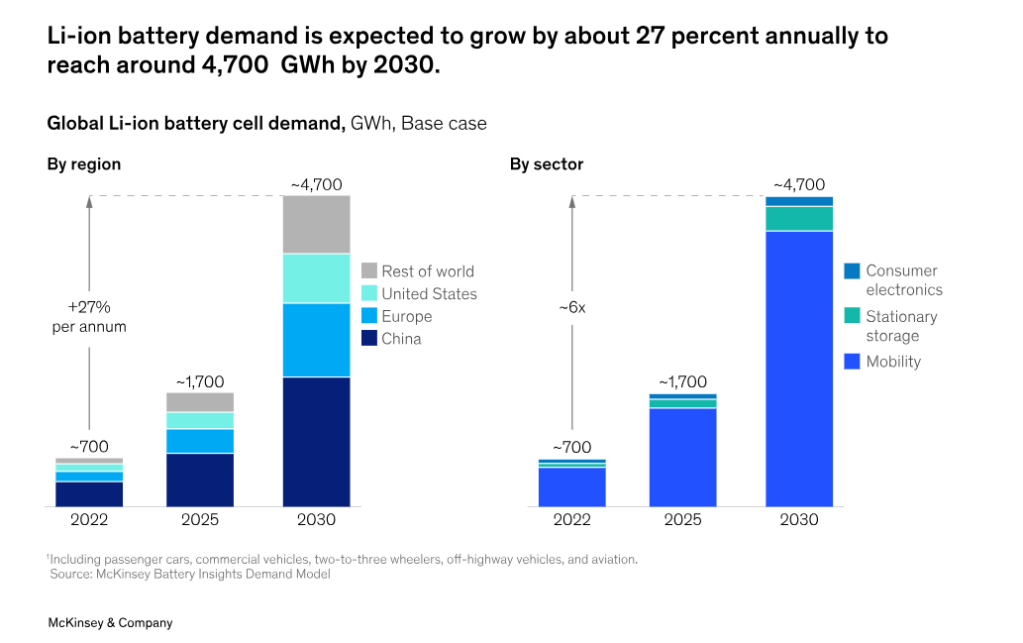
According to the Mckinsey 2023 report (Battery recycling takes the driver’s seat), across the battery recycling value chain, from collection to metal recovery, revenues are expected to grow to more than $95 billion a year by 2040 globally, predominantly driven by the price of the recovered metals, expected battery cell chemistry adoption, regionalization of supply chains, etcetera.
Balancing Electrification Goals: Addressing Human Costs
The global cobalt market is highly concentrated with the top five countries supplying >80% of the global market. The Democratic Republic of the Congo (DRC) alone supplies 71% of the global market, highlighting the dependence the cobalt market has on one country to supply, and keep on supplying, this strategic metal. But along with this we should also note that The Democratic Republic of the Congo is one of the poorest and most corrupt countries in the world. The DRC has had more deaths from war than any other country since World War II.
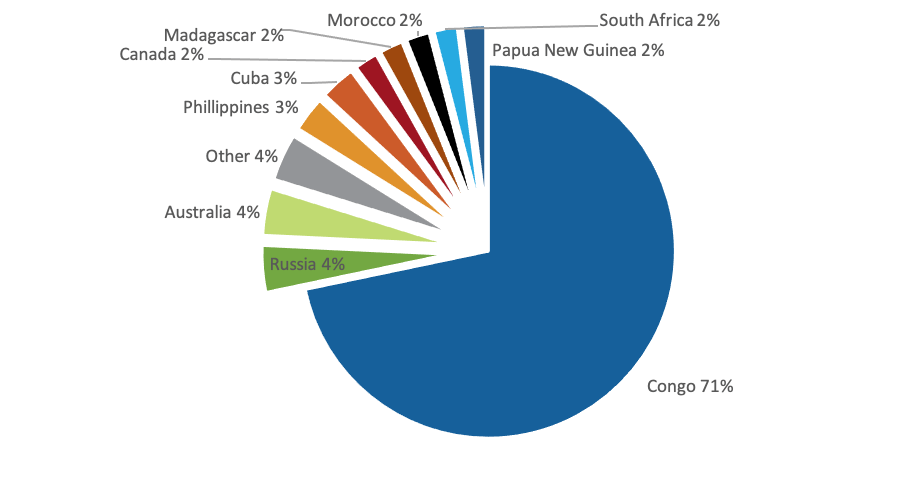
About 15% of the DRC’s cobalt is produced in small-scale, artisanal mines. Artisanal miners are freelance workers who independently extract cobalt and sell it on the informal market, earning less than $10 USD a day. Working at artisanal mines is associated with the risk of injury or death. This is because miners do not have basic protective equipment like hard hats and vests, and they usually work barefooted and hand-dig ores.

All the above focus on e-waste recycling, which will in turn help meet the rising demand for cobalt and stem the environmental degradation and social pressures the mining brings. There is a critical inflection point ahead for lithium-ion batteries as demand will potentially outpace the supply of key metals, so recycling must play a critical role in bridging this gap. Resource conservation, environmental impact, and cost-effectiveness will be key drivers for accelerating the innovation in the future. But it is also a fact for all people at the consumer end to think that ‘Future is Green & Electric. But at what cost?’
“If you care about the environment and how it will affect mankind, then recycling shouldn’t take a second thought.”
– Jeffrey Sanderson
Great topic and a very well-written article Vedant. In the United States, the Environmental Protection Agency (EPA) has set a goal of recycling 99% of all spent batteries by 2030. This goal is ambitious, but it is achievable with the right policies and infrastructure in place. This shows the importance and the opportunities. I cannot wait for your next article.
LikeLiked by 1 person
Thanks a lot, Arash for your feedback!!
LikeLike